A breast cancer vaccine could be closer to reality, according to the Cleveland Clinic, as researchers have announced some encouraging results.
At the Society for Immunotherapy of Cancer earlier this month in Texas, researchers shared updated findings from a study on a new vaccine designed to target triple-negative breast cancer, a press release said.
Triple-negative breast cancer (TNBC) is a very aggressive type of breast cancer, according to the American Cancer Society (ACS).
4 hidden signs of breast cancer: ‘You know yourself’
TNBC grows and spreads faster than less aggressive types and is more difficult to treat.

G. Thomas Budd, MD, lead study investigator, is pictured in the Cleveland Clinic lab. “Investigational vaccines are a potential new way to fight breast cancer,” he said. (Courtesy of Cleveland Clinic)
The Cleveland Clinic’s researched breast cancer vaccine is the first aimed at preventing triple-negative breast cancer, according to G. Thomas Budd, MD, principal investigator of the phase 1 study at the Cleveland Clinic Cancer Institute.
AFTER A BREAST CANCER DIAGNOSIS, THESE ARE 10 IMPORTANT THINGS TO DO, EXPERTS SAY.
The vaccine uses a protein found in breast tissue dedicated to lactation — called α-lactalbumin — that is no longer made after a woman reaches puberty, Budd told Fox News Digital.
“Using retired lactation proteins as breast cancer vaccine autoantigens makes sense because most breast cancers occur in women 40 and older, and many of these women are no longer breastfeeding,” he said.

Researchers are conducting breast cancer vaccine-related experiments in a laboratory at the Cleveland Clinic. The vaccine uses a protein found in breast tissue dedicated to lactation, called α-lactalbumin. (Courtesy of Cleveland Clinic)
This protein was chosen because it is no longer found in detectable amounts in normal, aging breast tissue, but is expressed at high levels in more than 70% of triple-negative breast cancers, Budd shared.
“Investigational vaccines are a potential new way to fight breast cancer,” he said.
“This represents a paradigm shift in the way cancer treatment is approached – a focus on prevention rather than treatment after the fact.”
In the Phase 1 trial, researchers found that the investigational vaccine was “generally tolerated and produced an immune response in most patients,” according to a Cleveland Clinic press release.
The team also presented vaccine side effects, best tolerated doses and immunological effects, the release said.
‘I’M A RADIOLOGIST – To reduce your risk of BREAST CANCER, eat these 5 foods and follow these healthy habits’
The Phase 1 trial, funded by the US Department of Defense, is being conducted at the Cleveland Clinic’s main campus in partnership with Anixa Biosciences, Inc., a California-based biotechnology company focused on treating and preventing cancer.
It included 26 patients in three separate groups.
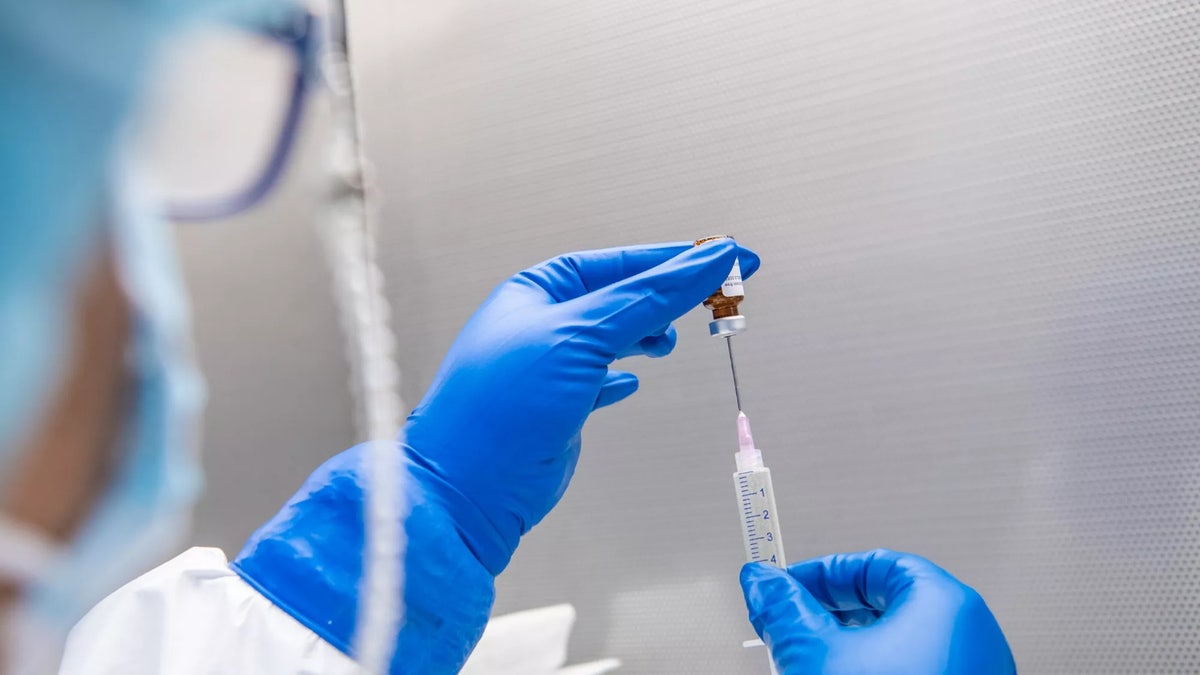
A pharmaceutical research technician demonstrates the preparation of a new breast cancer vaccine. (Courtesy of Cleveland Clinic)
The findings come after nearly two decades of research by the late Vincent Tuohy, PhD, of the Cleveland Clinic Lerner Research Institute, Budd noted.
Next year, Anixa plans to launch a Phase 2 study to measure the vaccine’s effectiveness.
“Our hope is that future studies will demonstrate that the antigen-specific T cell responses we observed translate to prevention of triple-negative breast cancer recurrence,” Budd said.
CLICK HERE TO SIGN UP FOR THE HEALTH NEWSLETTER
Fox News medical contributor, Dr. Nicole Saphier, called the development of a breast cancer vaccine “groundbreaking and exciting.”
“This represents a paradigm shift in how we approach cancer treatment — a focus on prevention rather than treatment after the fact,” Saphier, who was not involved in the research, told Fox News Digital.

A bottle of breast cancer vaccine is pictured in a laboratory. “The whole cancer community is waiting for further progress in this area, because it can start a new era of cancer prevention strategies,” said the doctor. (Courtesy of Cleveland Clinic)
“If successful, the vaccine could reduce the incidence of breast cancer, save countless lives and reduce the emotional, physical and financial burdens associated with cancer treatment.”
CLICK HERE TO GET THE FOX NEWS APP
The development of a breast cancer vaccine is very important for people at high risk, the doctor noted – “but it also offers the potential for wider public health benefits, helping to reduce the social and economic impact of cancer.”
For more Health articles, visit www.foxnews.com/health
Saphier added, “The whole cancer community is looking forward to further progress in this area, as it could create a new era of cancer prevention strategies.”




